Juniper Publishers: Role of Endometriosis in Infertility and Embryonic Loss: More than Anatomical Reasons
JUNIPER PUBLISHERS- JOURNAL OF GYNECOLOGY AND WOMEN’S
HEALTH
Journal of Gynecology and Women’s Health-Juniper
Publishers
Authored by Rudy Leon De Wilde*
The association between endometriosis and infertility
is widely recognized, but several studies demonstrate that
endometriosis also affects pregnancy rates in different ways other than
anatomical distortion of internal reproductive organs; however the
evoking mechanisms are complex. In mild to advanced stages of disease,
peritoneal adhesions, tubal obstruction, destruction of ovarian tissue
or distortion of the uterine wall are evident originating factors of
infertility or miscarriage. In contrast, in 17% of all cases of
endometriosis, the reason of infertility is not clear [1],
especially in women less than 35 years-old, who have a 2-fold increased
risk of unexplained infertility (HR= 2.12, 95% CI= 1.76-2.56) [2],
although miscarriage rate after ICSI is not age dependent (<35
years-old vs.> 35 years-old women; OR 0,4; 95% CI 0,1-2,1) [3].
The underlying abnormalities could explain the modest pregnancy rates
achieved by currently available medical and surgical treatments,
estimated in 52.9-83% in mild disease and 0-6,7% insevere disease [1].
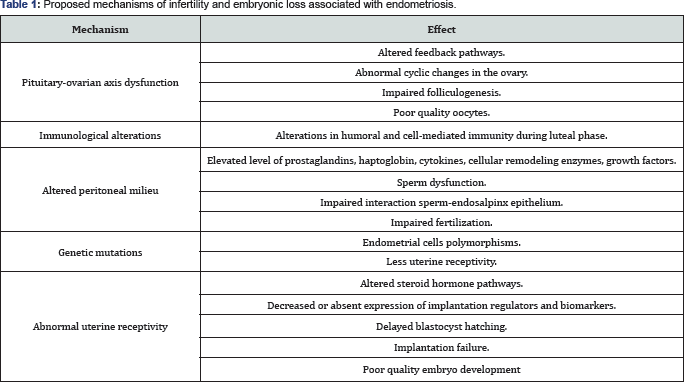
Some of the known pathophysiologic factors implied in endometriosis-associated infertility are irreversible (Table 1).
They generate functional and structural abnormalities of the
hypothalamic-pituitary-ovary-endometrium axis, which in turn lead to
retardation of sperm, oocyte and blastocyst transport, alteration of
endometrial receptivity and, finally, embryonic loss.
Hormonal dysfunction in endometriosis includes [4,5]:
extended follicular phase with aberrant patterns of LH-secretion, lower
levels of circulating estrogen, androgen and progesterone, and
increased intrafollicuaractivin, cytokines and growth factors. This in
turn induces premature apoptosis of cumulus cells, alteration of the
morphology, maturation and liberation of oocytes, and impairsoocyte
fertilization potential. At ultrasound, impairment of follicular growth
and low dominant follicle size could be observed. It is also possible to
observe trapped oocytes in a luteinizing corpus hemorragicum as a
signal of anovulation and altered corpus luteum development, defined as
luteinized unruptured follicle syndrome (LUFs).
The local immunologic alteration induced by
endometriosis results in an inappropriate milieu for sperm, oocytes,
embryo development and uterine receptivity [5].
This milieu is characterized by a high concentration of inflammatory
cytokines, oxidative stress products and reactive oxygen species (ROS).
These substances affect spermatozoa membrane, leading to an impaired
sperm function or rupture, including abnormal sperm- endosalpinx and
sperm-oocyte interaction, low number of free spermatozoa in tubal
ampulla, and loss of sperm fertilization potential.
Women with endometriosis also have six-times more
nuclear and cytoplasmic aberrations than other women and have been
associated with disease severity [6].
These aberrations include polymorphisms, dysregulated micro-RNAs and
epigenetic factors. Such factors are able to induce DNA hypomethylation
with subsequent alteration of endometrial receptivity mediators and
implantation failure [7].
Defects in blastocyst implantation could result due
to altered hormonal levels, embryo anomalies orprogesterone-target genes
dysregulation [7].
The last leads to a local progesterone resistance and to an
inhospitable environment, which in turn impairs blastocyst implantation.
This environment is characterized by a decreased or absent expression
of implantation and regulators markers of endometrial receptivity, such
as integrins, glycodelin A, leukemia inhibitory factor, osteopontin and
lysophosphatidic acid receptor. Although hormone and inflammatory marker
levels, and uterine cavity anatomy can easily be studied, there is
insufficient evidence to recommend an appropriate assessment of
endometrial receptivity.
Some studies report that progesterone supplementation
and endometrial biopsy may improve endometrial receptivity and
pregnancy rates, especially in IVF-embryo transfer cycles [8].
However, the analysis of published evidence is difficult given the
quality of the studies and the diversity of proposed treatments,
especially those involving assisted- reproductive techniques.
Progesterone administration for luteal phase support in embryo transfer
protocols is effective when given on the day or the day- after oocyte
pick-up (OR 1.87; 95% CI 1.13 to 3.08). On the other hand, increased
endometrial implantation competency after endometrial biopsy are based
on endometrial wound healing mechanisms, including secretion of
cytokines and growth factors accompanied of stem cells recruitment,
which are free of epigeneticdefects, favoring embryo implantation.
Treatments with not conclusive efficacy are systemic administration of
heparin, aspirin, prednisone, immunoglobulins or recombinant
follicle-stimulant hormones. Future therapies involving immunomodulators
or hormonal suppressive therapies could be useful to improve fertility
and pregnancy rates [9].
Conclusion
As physicians and gynecologists, the better we
understand the underlying causes of infertility, failure of uterine
receptivity and impaired embryonic implantation associated to
endometriosis, the more appropriate and secured therapies we can
provide, although many of these pathophysiological mechanisms are right
now not influenceable. Therefore more research is needed to design
specific therapies capable of modulating the changeable ones, to improve
fertility rates, and to reduce embryonic loss rates, especially when we
are facing patients undergoing assisted-reproductive treatments.
For more open
access journals in JuniperPublishers please click on: https://juniperpublishers.com/
For more articles on Gynecology and Women’s
Health please click on: https://juniperpublishers.com/jgwh/




Comments
Post a Comment