Juniper publishers: In Vitro Fertilization Turns 40: Highlights in Time Travel
JUNIPER PUBLISHERS- JOURNAL OF GYNECOLOGY AND WOMEN’S HEALTH
Journal of Gynecology and Women’s Health-Juniper Publishers
Authored by Jan Tesarik*
Abstract
In vitro fertilization (IFV) emerged as a relatively simple technique and served only in a few clinical indications related to infertility. Nowadays, techniques derived from IVF employ a huge spectrum of methodologies, are used in a wide range of indications, including those not related to infertility, and have interfaces with many fields of modern biology and medicine. This mini review resumes the fascinating travel of IVF over the last four decades, highlights the main break through that marked the development of IVF to its current state-of-the-art and traces paths of further evolution.
Keywords: In vitro fertilization; Controlled ovarian stimulation; Embryocryo preservation; Gamete intra fallopian transfer; Intra cytoplasmic sperm injection; Spermatid conception; Nuclear and cytoplasmic transfer; Artificial gametes; Artificial uteri
The Beginning
The concept of In Vitro Fertilization (IVF) goes back more than a century, but the first childbirth was only achieved in 1978 [1]. This event was at the origin of a spectacular evolution of IVF- related techniques over the last 40 years (Table 1).
The first birth after IVF [1] was achieved with a relatively simple methodology, involving oocyte recovery in a spontaneous ovulatory cycle, and allowing fertilization to occur spontaneously during oocyte incubation with spermatozoa in the laboratory The technique whereby IVF is achieved spontaneously, by active penetration of the fertilizing spermatozoon in to the oocyte, is now called conventional or classical IVF, to distinguish it from modes of IVF using different kinds of assistance to the fertilization process.
From the beginning till the end of the eighties
Soon after the birth of the first IVF baby it became evident that the technique could benefit from increasing the number of oocytes available in each treatment attempt. This led to the introduction of controlled ovarian stimulation which also simplified the timing of oocyte recovery from the ovary (Table 1). The first stimulated IVF cycles involved the use of human menopausal gonadotropin (HMG), for promotion of follicle growth, followed by human chorionic gonadotropin (HCG) to induce final oocyte maturation [2].
In its beginnings IVF was being accepted with some doubts and skepticisms. Some currents of thinking raised doubts about safety of extra corporeal fertilization, while other sargued about allegedly un natural way of reproduction, not worthy of human beings. To make the issue more acceptable for the most conservative sectors of the society, gamete intra fallopian transfer (GIFT) was invented in 1983 [3]. This technique, alowing for natural fertilization to take place in the fallopian tube after separate transfer of spermatozoa and oocytes (Table 1), was later adapted for the laparoscopic approach [4] and was being performed for some time as an alternative to conventional IVF.
In the same year 1983, some other important innovations appeared both at the laboratory and the clinical level (Table 1). In the laboratory, embryologists were confronted with the challenge of making the maximal use of multiple oocytes recovered in a single treatment cycle as results of controlled ovarian stimulation. This demand encouraged the adaptation of the technique of embryo cryopreservation, previously shown to be effective in non-human mammalian species, to be used with human embryos, resulting in the first childbirths [5]. However, some of the mutiple oocytes recovered after controlled ovarian stimulation are often immature and cannot be used for IVF immediately. This led to the development of the technique of human oocyte in vitro maturation and the first birth after IVF of in vitro matured human oocytes [6]. In addition to enhancing the economy of oocytes recovered after a single cycle of controlled ovarian stimulation, the technique of in vitro maturation later became important in the context of some techniques using nuclear transfer to immature oocytes (Table 1).
The clinical advancements achieved in the field of IVF in 1983 were no less important than those concerning the laboratory work. The use of vaginal route of ovarian follicle aspiration under ultrasound guidance [7] simplified the oocyte recovery procedure and was the first step towards making IVF a more patient-friendly procedure (Table 1). The application of human chorionic gonadotropin (HCG) after embryo transfer [8] marked the beginning of the development of treatment protocols aimed at enhancing embryo implantTable 1). The importance of these protocols in assisted reproduction is now widely accepted, and similar treatments have been proposed even in cases of natural conception, for women who suffer early pregnancy loss due to luteal phase insufficiency [9].
In the following years two important breakthroughs have been reported: the first successful assisted reproduction attempt with donated oocytes [10] and the first birth after embryo biopsy for pre implantation genetic analysis [11]. Even though oocyte donation was originally intended for the use in women with primary ovarian failure, the indications have rapidly expanded to other conditions of compromised oocyte quality (Table 1). Nowadays, a great part of oocyte donation treatment attempts is performed in women with physiological menopause.
In its turn, the first embryo biopsy for pre implantation genetic analysis [11] has opened the era of pre implantation genetic diagnosis (PGD) and pre implantation genetic screening (PGS). PGD, originally used to determine the sex of embryos in order to avoid the birth of children with sex-linked genetic diseases, has later been applied to a huge variety of singlegene mutations responsible for recessive genetic disorders to distinguish between embryos which would give rise to affected children, to children carrying the mutated gene but not affected and those free of the mutation. More recently, biopsied embryo cells have also been used to evaluate the whole genome for normal chromosome number or for a gain or loss of chromosomal material, without looking for a concrete gene defect. This latter technique is now commonly called PGS. Quite recently it has been shown that PGS can be performed in a noninvasive way, without embryo biopsy, by sequencing the genomic DNA released by human in vitro grown embryos into the culture medium [12].
The Nineties
The 1990 decade started with an important clinical innovation - the introduction of recombinant pituitary hormones to controlled ovarian stimulation protocols (Table 1). After the first successful IVF treatment with the use of recombinant human FSH [13], other recombinant hormone preparations gained popularity [14], particularly because of their standard properties and availability independent of biological raw materials from which hormones were isolated and purified before. Nowadays, most of hormonal preparations used in controlled ovarian stimulation worldwide are recombinant.
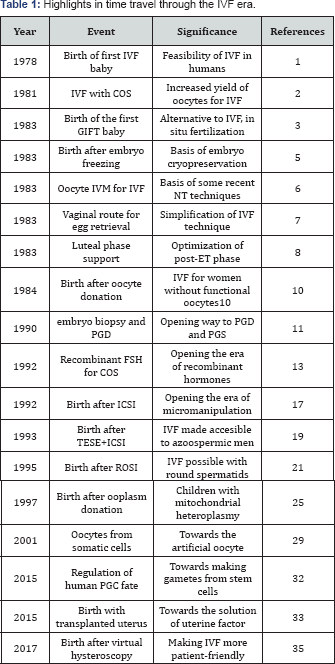
However, the principal breakthroughs of the 1990s were achieved in the laboratory and were related to the application of new cell micromanipulation technologies. The story began with attempts at assisting sperm-oocyte fusion by depositing spermatozoa in the narrow space between the oocyte and the zona pellucida (perivitelline space) by means of a micromanipulation needle. This technique, known as subzonal insemination (SUZI), was used in cases of severe male factor infertility in which spermatozoa were failing to penetrate the zona pellucida by its own means. Even though births were achieved after fertilization by SUZI [15], the technique circumvented the physiological block to oocyte polyspermic penetration and, in fact, the technique was burdened by a high incidence of polyspermy [16] and was progressively abandoned. The next and decisive step in the development of micromanipulation-assisted fertilization was a direct intracytoplasmic sperm injection (ICSI) whereby a single spermatozoon is injected into the oocyte cytoplasm [17]. The use of ICSI for oocyte fertilization allowed men with extremely impaired sperm number and function to father a child, including cases of complete lack of sperm movement. In addition to this revolutionary clinical innovation, ICSI also opened new perspectives in the study of the early biological reactions of the oocyte to the presence of the fertilizing spermatozoon (Figure 1) and the role of these reactions in the control of subsequent embryonic development [18]. Subsequent studies into these mechanisms opened a new era in developmental biology, enabling a deeper understanding of the causes of embryo aneuploidy and other abnormalities and opening alternative ways of embryo creation (Table 1).
The years following the first success of ICSI were marked by further extensions of the same gamete micro manipulation technique to new clinical scenarios (Table 1). To begin with, the fact that fertilization by ICSI did not require sperm movement facilitated fertilization with immature spermatozo is covered by testicular biopsy [19]. But researchers went further back stream the spermatogenic line. Encouraged by the finding that normal young can be achieved by fertilizing mouse oocytes with round spermatids [20], round spermatid injection (ROSI) was used to fertilize oocytes in cases of men with postmeiotic sperm maturation arrest, leading to the birth of healthy babies[21] . Another success of ROSI was reported three years later [22] , but the largest clinical series of ROSI babies was published recently [23]. Childbirth has also been achieved by fertilizing oocytes with round spermatids developing in vitro from primary spermatocytes from men with premeiotic sperm maturation arrest [24].
In the late 90s, the first children were born after fertilization of oocyte contructs originating from two different women, leading to mitochondrial heteroplasmy (Table 1). In addition to the original technique, using injection of anucleate donor oocyte cytoplasm to recipient oocytes [25] an alternative technique using meiotic chromosome transfer from mature human oocytes to donor cytoplasts (previously enucleated oocytes) was developed [26]. This latter approach makes it possible to create embryos with a high prevalence of the donated cytoplasm, which makes it advantageous for generating healthy embryos for women whose oocytes would otherwise transmit a mitochondrial disease [27], a technique widely divulged recently as the “3-parent” story [28].
The 21st Century
Several trends dominated the evolution of IVF in the first decade of 2000. Efforts to preserve fertility in different clinical scenarios marked one of them. In 2002 the first embryos were obtained by fertilizing human oocytes reconstructed from the patient's somatic cells and enucleated donor oocytes [29]. In parallel, fertilization of oocytes with somatic cells instead of spermatozoa was achieved in the mouse model [30]. The human embryos resulting from the reconstituted oocytes [29] have not been transferred to the uterus, for fear of chromosomal abnormalities. In fact, analysis of mouse oocytes derived from adult somatic cells showed abnormal arrangement of chromosomes on metaphase spindles in most cases [31]. Notwithstanding, these works marked the trend towards the development of artificial gametes to be used for fertilization in cases in which the male or the female germline is irreversibly lost (Table 1).
The first two decades of 2000 were characterized by further refinements of the concepts created previously. This concerned both the controlled ovarian stimulation protocols and laboratory work. With respect two the previously mentioned project of artificial gamete creation, an important breakthrough was achieved recently by a study identifying the epigenetic mechanisms which can determine reprogramming of human stem cell-derived primordial germ cells towards the male or the female genomic imprinting pattern [32]. This research is crucial to the development of techniques by which stem cell nuclei can be used to substitute sperm and oocyte nuclei in fertilization (Table 1). The absence of the uterus or the failure of uterine receptivity is other problems of female reproduction which are approaching the solution. In 2015 the first live birth after uterus transplantation has been reported [33]. Moreover, the recent publication of an extrauterine system to physiologically support the extreme premature lamb [34] awakens a hope that the complete development of human embryos outside the maternal organism will be possible one day.
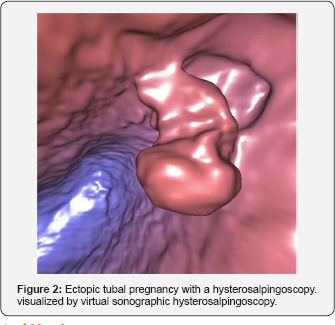
Last but not least, a clear trend towards making IVF and the associated diagnostic examinations more patient-friendly is evident in the last years. In particular, examinations which can produce pain or patient discomfort are progressively shifted from the operating theater towards virtual reality. This is the case of some endoscopic examinations, such as hysteroscopy and hystero salpingoscopy which can now be replaced by a simple vaginal sonographic examination followed by computerized 3-dimensional reconstruction of the cavities and canals examined [35,36]. The same technique can be used to virtually penetrate spaces which are not accessible to direct endoscopy (Figure 2).
And Next?
Well, dear time travelers. We are now here, but the journey is going on. We have revisited its most fascinating moments, but the path is paved by many other excellent pieces of work which are not mentioned here and yet were substantial to get us where we are. In conclusion, the 40 years elapsed from the first successful IVF till now were marked by continuous effort to enlarge its application field to new clinical scenarios and to make it more efficient, more secure and more patient-friendly. Further research is expected to resolve remaining challenges, such as artificial oocytes, artificial spermatozoa and artificial uteri.
For more open access journals in JuniperPublishers please click on: https://juniperpublishers.com/
For more articles on Gynecology and Women’s Health please click on: https://juniperpublishers.com/jgwh/
To read more......Fulltext in Gynecology and Women’s Health in Juniper Publishers
https://juniperpublishers.business.site/




Comments
Post a Comment