Juniper Publishers : Uterine Serous Carcinoma; Molecular Pathways and Role of Micrornas in Early Detection and Target Therapy
JUNIPER PUBLISHERS- JOURNAL OF GYNECOLOGY AND WOMEN’S HEALTH
Journal of Gynecology and Women’s Health-Juniper Publishers
Authored by Shiva Rezaei*
Abstract
Uterine serous carcinoma (USC) in association with endometrial clear cell carcinoma (CCC) and grade 3 endometrioid carcinoma is responsible for 70-75% of endometrial carcinoma deaths and per se accounts for 39% of this cancer related mortality. Its tendency for early spread, leads to upstaging of 50-70% of clinically stage I cancers at the time of operation. Involved molecular pathway(s) in this cancer differ from those of conventional endometriod carcinoma. It has been accepted that P53 mutation occurs as an early event in the endometrial serous carcinogenesis. Findings of P53 signature in the endometrial polyps and minor epithelial atypia (Endometrial Glandular Dysplasia- EmGD) supported this assumption that mutation in PT53 may develop before apparent morphologic atypia (p53 signature), or occur in pre-existing malignant lesions of endometrium such as high grade endometriod carcinoma. Early detection through minimally-invasive approaches appears to be the gold strategy decreasing the uterine serous carcinoma related mortality. During last decades researchers have been interested in discriminating the specific and sensitive biomarkers such as microRNAs for early detection of invasive cancers. These recently explained microRNAs can be useful in monitoring the tumor response against different therapeutic agents as well as in tracing of disease process. In this review firstly we will concentrate on clinical aspects of uterine serous carcinoma and the essential molecular pathways of serous carcinogenesis .Then the main microRNAs involved in development of this cancer comprehensively will be discussed.
Keywords: Uterine serous carcinoma; Molecular pathways; MicroRNAs
Abbrevations: USC: Uterine Serous Carcinoma; EIC: Endometrial Intraepithelial Carcinoma; CCC: Clear Cell Carcinoma; mTOR: Mammalian Target of the Rapamycin; EMT: Epithelial Mesenchymal Transition
Introduction
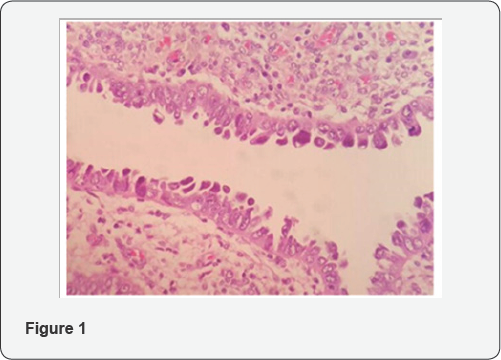
As a high grade tumor, uterine serous carcinoma (USC) accounts for less than 10% of endometrial carcinomas (EC), but in association with endometrial clear cell carcinoma (CCC) and grade 3 endometrioid carcinoma is responsible for 70-75% of EC deaths [1] and per se accounts for 39% of this cancer related deaths [2]. As a prototype of type II endometrial carcinoma, USC is an aggressive disease and near 60-70% of the patients present with extrauterine spread at the time of diagnosis [2]. In contrast with more common type I endometrial carcinoma, serous carcinoma tends to occur in older age group and usually diagnosed at advanced stages [3]. Even among women who have not myometrial invasion, 33-50% of them will show extrauterine spread in comprehensive staging [4]. Its tendency for early spread, leads to upstaging of 50-70% of clinically stage I cancers at the time of operation [5]. Presentation of 46% of patients with USC and endometrial clear cell carcinoma in stage II-IV compared to 21% for all endometrial cancers confirms the common perception that these histotypes carry a worse prognosis due to advanced disease at the time of diagnosis [6]. Although the term "Endometrial Intraepithelial Carcinoma”(EIC) is widely used to designate the precursor lesion of uterine serous carcinoma, it should be acknowledged that "early serous carcinoma” or other alternative diagnostic terms have been suggested for use in clinical practice, in recognition that some serous carcinomas without invasion in the uterus are associated with extra-uterine spread [7,8]. For this reason Clement and Young considered EIC (Figure1) as a tiny focus of serous carcinoma and did not qualify it further other than to note its size and location and stressed that pathologists should indicate its malignant potential , in the pathology report when it is unaccompanied by typical serous carcinoma [9].
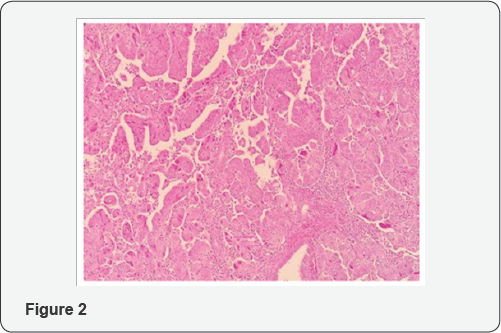
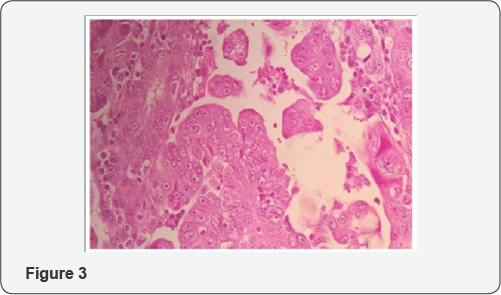
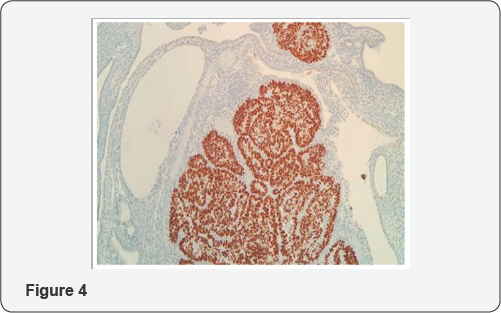
From histologic standpoint, USC presents as a high grade malignant tumor and shows slit like glands, short and thick papillary structures or solid aggregation of malignant epithelial cells (Figure 2). In high magnification the neoplastic cells have notable pleomorphic nuclei, prominent nucleoli and numerous mitosis including atypical types (Figure 3). It has been widely accepted that USC evolves via molecular genetic pathway(s) that differ from those of conventional endometriod carcinoma [10-15]. Although the dualistic module proposed by Bochman [16] has been conceptually useful and provided a framework for other important works, it is unable to explain these diversities in term of histology and molecular change in each of subgroups. Numerous studies proposed that P53 mutation occur as an early event in the endometrial serous carcinogenesis [17-20] (Figure 4) and based on a recent study on latent precursor (P53 signature) in the endometrial polyps [21], it has been suggested that the mutation in PT53 may develop before apparent morphologic atypia (p53 signature), associate with minor epithelial atypia (EmGD) or occur in pre-existing malignant lesions of endometrium ( high grade endometriod carcinoma) resulting in biphasic tumor pattern.
Early detection through minimally-invasive approaches appears to be the gold strategy decreasing the USC related mortality .During last decades researchers have been interested in discriminating the specific and sensitive biomarkers such as microRNAs detectable by simple methods. These recently explained microRNA can be useful in monitoring the tumor response against different treatments as well as in tracing of disease process [22]. However, the early stage diagnosis of cancers by microRNAs is still in its infancy
MicroRNAs are small non-coding RNAs play special roles in post-transcriptional regulation of genes and are a large class of noncoding RNAs evolutionarily conserved in mammals [23]. Detection of miRNAs can be directed through various approaches including qRT-PCR, microarray and sequencing [24,25]. Accumulating studies have been conducted to define a valuable pattern of micoRNAs to improve early diagnostic and prognostic approaches. Accordingly, researchers from all over the world investigated microRNAs profiling in endometrial serous carcinoma patients. Current review has focused on the main genetic pathways involved in uterine serous carcinogenesis and latest literatures on microRNAs biomarker discovery validated for USC.
1-Molecular Carcinogenesis in Uterine Serous Carcinoma
Endometrial Serous Carcinomas commonly show mutation in p53, over-expression of cyclin E and HER2/neu, p16 dys- regulation, genetic alternations in E-cadherin and aberrations within the PI3K pathway [26]. A genome-wide analysis directed by Kuhn and coworkers showed that disruptions in p53, PI3K, and cyclin E pathways, were significantly detected in uterine serous carcinomas [27].
P53
Mutation in p53 is the most characteristic genetic alteration of non endometriod endometrial carcinomas [28]. As a tumor suppressor gene, p53 is located in 17p13.1, encodes the nuclear phosphoprotein p53 [29], and has important roles in preventing of inappropriate cell proliferation and maintaining the genome integrity following genotoxic stresses [30]. Loss of p53 function leads to apoptosis inhibition [31] and mutations in p53 can trigger signaling for transforming growth factor b (TGF-b) receptor, epidermal growth factor receptor, and mesenchymal epithelial transition (MET) [32,33].
The level and activity of p53 is regulated by a complex network of several proteins such as HPV16 E6, WT-1, E1B/E4, SV40 T-antigen, MDM2, JNK, Pirh2, and PARP-1 [34]. Findings suggest that there are significant associations between advanced stages and high grade tumors with p53 mutations resulting in frequently poor outcome [35]. One hypothesis suggests that mutation in one allele occurs early during the development of serous carcinoma's precursor, meanwhile, normal second allele deficiency directs the progression toward serous carcinoma [36]. Another hypothesis discusses that serous carcinoma may be evolved through p53 mutation in previous endometrioid carcinoma based on presence of mixed endometrioid and serous carcinomas [37].
P16 (CDKN2A)
P16 located on chromosome 9 (9p21) [38], acts as a tumor- suppressor and negative regulator of the cell cycle [39] which is suggested as an critical marker to discrimination of uterine adenocarcinoma subtypes [40]. P16 inactivation was detected in approximately 45% of serous carcinomas. However, it is still not entirely clear what mechanism is involved in inactivation of P16 [41].
PI3K pathway
The phosphatidylinositol-3-kinase -AKT - mammalian target of the rapamycin (mTOR) pathway, an important mediator of different cell functions including the regulation of apoptosis, cell growth and proliferation, more frequently are disrupted in human malignancies [42]. The more common mutations in PI3K pathway are associated with PTEN, PIK3CA, and PIK3R1 [43-45].
Wilms tumor 1 gene (WT1)
The WT1 is a tumor suppressor gene located on the short arm of chromosome 11 at p13 [46]. There is a significant direct correlation between WT1 expression and histological grade as well as a trend toward worse clinical prognosis [47].
E-cadherin
E-cadherin as a key member of adhesion factors has an important role in cell polarity and integrity preservation and encoded by CDH1 gene [41,48]. It has extracellular Ca2+ binding sites to maintain intercellular stability by cell adhesion. In order to keep normal structure of the cells, the intracellular section of E-cadherin interplays with β-catenin and forms connections [49]. Reduced E-cadherin expression is associated with advanced stages in endometrial cancers [48]. Evidence showed that down- regulation of E-cadherin may be as a result of hypermethylation of promoter or Loss of heterozygosity [50].
HER2/neu
A multiple lines of evidences demonstrate that gene amplification of epidermal growth factor receptor II or HER2/ neu and its protein over- expression are associated with several human cancers including endometrial malignancies and poor prognosis [51]. This gene is defined as an essential oncogene has functions in signal transduction. HER2 up-regulation or amplification is more common among USC in comparison with endometrioid carcinoma [52-54] that comes along unfavorable prognostic factors.
Cyclin E
Cyclin E encoded by CCNE has key function in the regulation of G1 phase to S phase transition during cell cycle. Up-regulation of cyclin E leads to speed up in cell cycle through G1 phase by interplaying with cyclin-dependent kinase-2 (CDK-2). Findings suggested an important association between overexpression of cyclin E and endometrial carcinomas, although details of involved mechanisms remain to be elucidated. Cassia and coworkers for the first time reported that overexpression of cyclin E could be as a result of gene amplification which is more commonly detected in non-endometrioid endometrial carcinomas [55].
MicroRNAs Regulatory Function
MicroRNAs regulate the expression of mRNA either by its degradation or translation silencing, depending on the recognition of a target sequence within 3' UTR of mRNAs. MicroRNAs recognize a 6-7 nucleotide region in their target mRNAs complementary to the “seed” sequence of the microRNA. This seed site is determined between nucleotide positions 2 to 8 from the 5' region of the microRNA. In case of accurate base- pairing with the 3' UTR region of the mRNA target, microRNAs direct a cleavage in the target strand. Although, inaccurate complementary causes the inhibition of mRNA translation either at initiation or during elongation phases [22,56,57]. It should be pointed that a unique microRNA may have multiple mRNA targets. Also, a set of microRNAs may be included in the regulation of a single mRNA [58-60].
Function of microRNAs in cancer
Capability of microRNA to use as novel biomarkers: Detection of microRNA- based biomarkers will affect the diagnostic approach in personalized medicine dramatically. Lin- 4 was the first microRNA distinguished in C. elegans acting as down- regulator of LIN-14 [23]. Additionally, there are a number of reports suggesting the presence of microRNAs in body fluids e.g. blood, released by tumor cells [61-63].
For the first time, Lawrie and colleagues recognized the first blood- based microRNA associated with tumor in sera of B-cell lymphoma subjects [64]. Afterward, several groups have endeavored to detect specific and sensitive tumor secreted biomarkers related with different malignancy. On the other hand, microRNAs may be proper therapeutic agents holding promise as candidates for microRNA inhibition and replacement therapies in targeted treatments [65]. Also, they preserve essential features, such as special stability in body fluids, simple and pervasive determination approaches and more importantly the ability to screening and regular monitoring of the diseases and its response to therapies, encouraging the researchers in microRNA baesd- biomarker discoveries [66].
Further deep analysis suggest that tumor cells direct the signaling mediators like microRNAs in cell to cell communications and persuade transformation in the neighboring or distant regions. Accordingly, the cells more likely secrete distinct microRNAs and the other cells recognize and taken up them by molecular mechanisms remain to be fully discovered [67,68]. In this regard, the cells take special measures to keep microRNAs out of digestion by endogenous RNase including their association with RNA binding proteins like Argonaute2 [68].
Misregulation of microRNAs in cancers: The literatures reported both oncogenic and tumor suppressive functions for these group of noncoding RNAs. It was shown that microRNAs can disturb normal homeostasis of the cells and induce abnormality in various pivotal pathways ultimately lead to increased proliferation, promotion of angiogenesis, epithelial- mesenchymal transition, inhibition of apoptosis, invasion, metastasis, resistance against therapies and so on [69-71]. Moreover, argonaute2 [72-74] DROSHA and DICER [59-75] as critical components of the microRNA regulatory pathway, might be deregulated in some malignancies.
MicroRNA biomarkers detected in endometrial serous carcinoma during recent years: A number of Investigations have evaluated the possible relation between endometrial serous carcinoma and certain microRNAs. A study by Hiroki and colleagues suggested 54 down-regulated (such as miR- 101, miR-10b*, miR-29b and miR-152) and 66 upregulated microRNAs (including miR-205, miR-200a and miR-200b), could be considered as markers to discriminating endometrial serous carcinoma patients from normal cases. They also considered any significant relationship between differential expressions of miRNAs with clinicopathological characteristics of the patients. Their results demonstrated that down- regulation of miR- 10b*, miR-29b, and miR-455-5p detected in patients with more invasive stages, had key roles in cancer metastatic progression [76].
At the same time, another group reported that miR-22 is specifically upregulated in endometrial serous carcinomas compared to uterine carcinosarcomas but the expression of miR-182 is reduced [77]. In another study, Hiroki and coworkers showed that the expression of miR-34b decreased in comparison with normal subjects. Their findings suggested that CpG island hypermethylation is associated with down-regulation of miR- 34b expression [78].
In a more comprehensive study Devor, et al. provided the microRNA pattern ofendometrial endometrioid adenocarcinomas and serous adenocarcinomas. They significantly detected down- regulation of seven microRNAs and upregulation of 13 microRNAs in both groups. However, each type of endometrial cancer had also specific pattern of microRNA misregulation. For example, the expression of miR-155 and miR-370 significantly increased in serous adenocarcinomas in comparison with control cases (p=0.049 and p=0.024, respectively) [79]. A recent study, defined that miR-106b was down-regulated in highly invasive endometrial serous carcinoma cells. It could conduct the suppression of TWIST1 expression, as an essential mediator of epithelial mesenchymal transition (EMT), resulting in more invasive phenotypes of endometrial cancers [80].
In another investigation, the expression of miR-182 and its target (Cullin-5, a cullin-RING E3 ubiquitin ligase have function in various types of malignancies) were assessed by Devor and coworkers. They reported significantly reduction of Cullin-5 expression in both case groups (endometrioid and serous endometrial adenocarcinomas) compared with normal samples. Although, they showed that serous cancer subjects had more decline (-4.3-fold) than endometrioid types (-2.9-fold). They asserted that overexpression of miR-182 led to the reduction of Cullin-5 expression [81].
A more recent case- control study, exposed miRNAs pattern in African- American cases having uterine serous carcinoma and compared them with matched controls. The patients were followed- up for a medium time of 43 months. They declared that 649 micoRNAs had differential expression between tumor and normal subjects and presented miR-223 as a death risk factor. Their results displayed overexpression of miR-223 associated with disease recurrence and poor prognosis [82].
Conclusion
MicroRNAs as mediators of pivotal regulatory pathways have certain notable features awarding possibility to use them as novel diagnostic and prognostic biomarkers, targeted anti-cancer therapies and overcoming drug resistance. Despite the wide variety of microRNAs assumed to be involved in endometrial serous carcinomas, in some cases the inverse findings are remarkable from one study to another, more likely as a result of the inconsistency in sample preparation procedures, adopting the determination and normalization methods and especially the differentiation caused by genetic pools and environmental factors of initial subjects. As yet, the lack of a worldwide standard method to determination of microRNAs has been led to delays in its transition from lab to clinic.
Common treatments in combination with microRNA replacement and prophylactic strategies have obtained substantial promise to cure more invasive cancers. However, future studies should answer which panel of microRNAs has actually differential expression between endometrial serous carcinoma in comparison with normal controls and other types of endometrial carcinoma. Altogether, more prospective and comprehensive studies using standard procedures are necessary to exactly elucidation of microRNA- based biomarkers for early detection and predicting the invasive phenotypes.
For more open access journals in JuniperPublishers please click on: https://juniperpublishers.com/
For more articles on Gynecology and Women’s Health please click on: https://juniperpublishers.com/jgwh/
To read more......Fulltext in Gynecology and Women’s Health in Juniper Publishers
https://juniperpublishers.business.site/




Comments
Post a Comment